Atomic Force Microscopy
AFM basics
Atomic Force Microscopy (AFM) probes force interactions between a probe (AFM tip) and the sample, includes but not limited to, Van der Waals interactions, electrostatic interactions, capillary forces, other types of long and short range adhesive and repulsive forces (it should be noted that these interactions are not mutually exclusive). It detects the change of interactions by monitoring the deflection of laser focused on the cantilever (part of AFM probe), which then are used to map the surface topography through its feedback system that maintains those interactions constant.
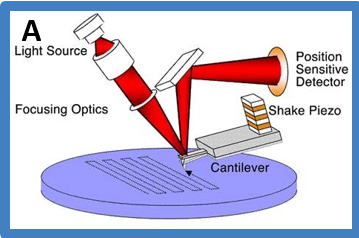 Mechanistic diagram of how AFM works
Mechanistic diagram of how AFM works
AFM images are intensity images. The Z values are intensity information such as height, amplitude, phase, etc. The following image is an example AFM height image that we obtained from experiments showing DNA-MutSβ interactions.
Due to the complexity of AFM techniques and sample constructs, AFM can be done with a substantial degree of flexibility and be used to probe different kinds of interactions. In addition to conventional AFM imaging on dried sample, our lab has also worked on imaging in fluid, electrostatic force microscopy (EFM) imaging, combined AFM-fluorescence imaging. Our lab and campus resources also enable us to pioneer cutting edge frontier of latest AFM researches such as fast imaging in biological native fluid environment.
DNA imaged in fluid using Cypher AFM. You can see distortions on part of the DNA during the video.
What can we learn from AFM?
- Protein-DNA Interactions:
- Binding affinities
- Binding specificities
- Stoichiometry
- Structural Features
- Protein-induced DNA bending and looping
- Protein-Protein Interactions:
- Binding affinities
- Stoichiometry
- Structural features of complexes
- DNA Conformations:
- Bending
- Supercoiling
- Looping
- Dual Resonance Frequency Enhanced Electrostatic Microscopy (DREEM):
- Protein and DNA electrostatic properties
- Visualize DNA path inside proteins
Our current and past studies using AFM can be found below, or navigated through the ‘Atomic Force Microscopy’ category on the left sidebar.
-
Posts related to AFM Studies
- Dual Resonance Frequency Enhanced Electrostatic Microscopy (DREEM)Dual Resonance Frequency Enhanced Electrostatic Microscopy (DREEM) is a variant of electrostatic force microscopy (EFM), which is an AFM based technique. A schematic illustration of the technique and sample images are shown below. Read more in the published article.More
- Combined AFM and Fluorescence Microscopy TechniqueAFM is a powerful technique for studying protein-protein and protein-DNA interactions. However, a significant limitation to AFM and other high-resolution microscopy is the difficulty of identifying different proteins in multi-protein complexes. To resolve this, we are interested in combining the ...More
- AFM Studies of DNA Repair – OverviewAFM provides us unique capability in revealing structural and functional relationship of DNA repair, including but not limited to, the specificity of the protein binding complex, the binding affinity, the DNA bending through bend angle analysis, the conformation analysis, and how ...More
- Dual Resonance Frequency Enhanced Electrostatic Microscopy (DREEM)

