Publications
2023
Rapid, inexpensive, sequence-independent fluorescent labeling of phosphorothioate DNA
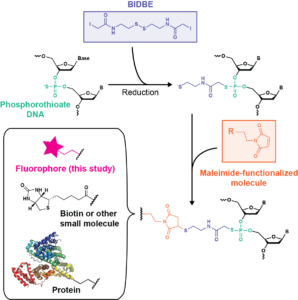
Fluorescently labeled oligonucleotides are powerful tools for characterizing DNA processes; however, their use is limited by the cost and sequence requirements of current labeling technologies. Here, we develop an easy, inexpensive, and sequence-independent method for site-specifically labeling DNA oligonucleotides. We utilize commercially synthesized oligonucleotides containing phosphorothioate diester(s) in which a nonbridging oxygen is replaced with a sulfur (PS-DNA). The increased nucleophilicity of the thiophosphoryl sulfur relative to the phosphoryl oxygen permits selective reactivity with iodoacetamide compounds. As such, we leverage a long-existing bifunctional linker, N,N′-bis(α-iodoacetyl)-2-2′-dithiobis(ethylamine) (BIDBE), that reacts with PS-DNAs to leave a free thiol, allowing conjugation of the wide variety of commercial maleimide-functionalized compounds. We optimized BIDBE synthesis and its attachment to PS-DNA and then fluorescently labeled the BIDBE-PS-DNA using standard protocols for labeling cysteines. We purified the individual epimers, and using single-molecule Förster resonance energy transfer (FRET), we show that the FRET efficiency is independent of the epimeric attachment. Subsequently, we demonstrate that an epimeric mixture of double-labeled Holliday junctions (HJs) can be used to characterize their conformational properties in the absence and presence of the structure-specific endonuclease Drosophila melanogaster Gen. Finally, we use a biochemical activity assay to show that this double-labeled HJ is functional for cleavage by Gen and that the double-labeled HJ allows multiple DNA species to be identified in a single experiment. In conclusion, our results indicate that dye-labeled BIDBE-PS-DNAs are comparable to commercially labeled DNAs at a significantly reduced cost. Notably, this technology could be applied to other maleimide-functionalized compounds, such as spin labels, biotin, and proteins. The sequence independence of labeling, coupled with its ease and low cost, enables unrestricted exploration of dye placement and choice, providing the potential for creation of differentially labeled DNA libraries and opening previously inaccessible experimental avenues.
2020
Recurrent mismatch binding by MutS mobile clamps on DNA localizes repair complexes nearby
PNAS
DNA mismatch repair (MMR), the guardian of the genome, commences when MutS identifies a mismatch and recruits MutL to nick the error-containing strand, allowing excision and DNA resynthesis. Dominant MMR models posit that after mismatch recognition, ATP converts MutS to a hydrolysis-independent, diffusive mobile clamp that no longer recognizes the mismatch. Little is known about the postrecognition MutS mobile clamp and its interactions with MutL. Two disparate frameworks have been proposed: One in which MutS–MutL complexes remain mobile on the DNA, and one in which MutL stops MutS movement. Here we use single-molecule FRET to follow the postrecognition states of MutS and the impact of MutL on its properties. In contrast to current thinking, we find that after the initial mobile clamp formation event, MutS undergoes frequent cycles of mismatch rebinding and mobile clamp reformation without releasing DNA. Notably, ATP hydrolysis is required to alter the conformation of MutS such that it can recognize the mismatch again instead of bypassing it; thus, ATP hydrolysis licenses the MutS mobile clamp to rebind the mismatch. Moreover, interaction with MutL can both trap MutS at the mismatch en route to mobile clamp formation and stop movement of the mobile clamp on DNA. MutS’s frequent rebinding of the mismatch, which increases its residence time in the vicinity of the mismatch, coupled with MutL’s ability to trap MutS, should increase the probability that MutS–MutL MMR initiation complexes localize near the mismatch.
Dynamic human MutSα–MutLα complexes compact mismatched DNA
Kira C. Bradford, Hunter Wilkins, Pengyu Hao, Zimeng M. Li, Bangchen Wang, Dan Burke, Dong Wu, Austin E. Smith, Logan Spaller, Chunwei Du, Jacob W. Gauer, Edward Chan, Peggy Hsieh, Keith R. Weninger, & Dorothy A. Erie, PNAS
DNA mismatch repair (MMR) corrects errors that occur during DNA replication. In humans, mutations in the proteins MutSα and MutLα that initiate MMR cause Lynch syndrome, the most common hereditary cancer. MutSα surveilles the DNA, and upon recognition of a replication error it undergoes adenosine triphosphate-dependent conformational changes and recruits MutLα. Subsequently, proliferating cell nuclear antigen (PCNA) activates MutLα to nick the error-containing strand to allow excision and resynthesis. The structure–function properties of these obligate MutSα–MutLα complexes remain mostly unexplored in higher eukaryotes, and models are predominately based on studies of prokaryotic proteins. Here, we utilize atomic force microscopy (AFM) coupled with other methods to reveal time- and concentration-dependent stoichiometries and conformations of assembling human MutSα–MutLα–DNA complexes. We find that they assemble into multimeric complexes comprising three to eight proteins around a mismatch on DNA. On the timescale of a few minutes, these complexes rearrange, folding and compacting the DNA. These observations contrast with dominant models of MMR initiation that envision diffusive MutS–MutL complexes that move away from the mismatch. Our results suggest MutSα localizes MutLα near the mismatch and promotes DNA configurations that could enhance MMR efficiency by facilitating MutLα nicking the DNA at multiple sites around the mismatch. In addition, such complexes may also protect the mismatch region from nucleosome reassembly until repair occurs, and they could potentially remodel adjacent nucleosomes.
Fabrication of a Biocompatible Mica/Gold Surface for Tip-Enhanced Raman Spectroscopy
Xiao You, Clayton B. Casper, Emily E. Lentz, Dorothy A. Erie, & Joanna M. Atkin, ChemPhysChem
Tip-enhanced Raman spectroscopy (TERS) is a promising technique for structural studies of biological systems and biomolecules, owing to its ability to provide a chemical fingerprint with sub-diffraction-limit spatial resolution. This application of TERS has thus far been limited, due to difficulties in generating high field enhancements while maintaining biocompatibility. The high sensitivity achievable through TERS arises from the excitation of a localized surface plasmon resonance in a noble metal atomic force microscope (AFM) tip, which in combination with a metallic surface can produce huge enhancements in the local optical field. However, metals have poor biocompatibility, potentially introducing difficulties in characterizing native structure and conformation in biomolecules, whereas biocompatible surfaces have weak optical field enhancements. Herein, a novel, biocompatible, highly enhancing surface is designed and fabricated based on few-monolayer mica flakes, mechanically exfoliated on a metal surface. These surfaces allow the formation of coupled plasmon enhancements for TERS imaging, while maintaining the biocompatibility and atomic flatness of the mica surface for high resolution AFM. The capability of these substrates for TERS is confirmed numerically and experimentally. We demonstrate up to five orders of magnitude improvement in TERS signals over conventional mica surfaces, expanding the sensitivity of TERS to a wide range of non-resonant biomolecules with weak Raman cross-sections. The increase in sensitivity obtained through this approach also enables the collection of nanoscale spectra with short integration times, improving hyperspectral mapping for these applications. These mica/metal surfaces therefore have the potential to revolutionize spectromicroscopy of complex, heterogeneous biological systems such as DNA and protein complexes.
2019
Structure, function, and inhibition of drug reactivating human gut microbial β-glucuronidases
Kristen A. Biernat, Samuel J. Pellock, Aadra P. Bhatt, Marissa M. Bivins, William G. Walton, Bich Ngoc T. Tran, Lianjie Wei, Michael C. Snider, Andrew P. Cesmat, Ashutosh Tripathy, Dorothy A. Erie, & Matthew R. Redinbo, Scientific Reports
Bacterial β-glucuronidase (GUS) enzymes cause drug toxicity by reversing Phase II glucuronidation in the gastrointestinal tract. While many human gut microbial GUS enzymes have been examined with model glucuronide substrates like p-nitrophenol-β-D-glucuronide (pNPG), the GUS orthologs that are most efficient at processing drug-glucuronides remain unclear. Here we present the crystal structures of GUS enzymes from human gut commensals Lactobacillus rhamnosus, Ruminococcus gnavus, and Faecalibacterium prausnitzii that possess an active site loop (Loop 1; L1) analogous to that found in E. coli GUS, which processes drug substrates. We also resolve the structure of the No Loop GUS from Bacteroides dorei. We then compare the pNPG and diclofenac glucuronide processing abilities of a panel of twelve structurally diverse GUS proteins, and find that the new L1 GUS enzymes presented here process small glucuronide substrates inefficiently compared to previously characterized L1 GUS enzymes like E. coli GUS. We further demonstrate that our GUS inhibitors, which are effective against some L1 enzymes, are not potent towards all. Our findings pinpoint active site structural features necessary for the processing of drug-glucuronide substrates and the inhibition of such processing.
Ctp1 protein-DNA filaments promote DNA bridging and DNA double-strand break repair
Sara N. Andres, Zimeng M. Li, Dorothy A. Erie, & R. Scott Williams, Journal of Biological Chemistry
The Ctp1 protein in Schizosaccharomyces pombe is essential for DNA double-strand break (DSB) repair by homologous recombination. Fission yeast Ctp1 and its budding yeast (Sae2) and human (CtIP) homologs control Mre11–Rad50–Nbs1 nuclease complex activity and harbor DNA-binding and -bridging activities. However, the molecular basis for Ctp1–DNA transactions remains undefined. Here, we report atomic force microscopy (AFM) imaging of S. pombe Ctp1–DNA complexes revealing that Ctp1 polymerizes on dsDNA molecules and forms synaptic filaments that bridge two dsDNA strands. We observed that Ctp1 DNA filaments are typified by an average filament length of ∼180 bp of dsDNA and a Ctp1 tetramer footprint of ∼15 bp. Biochemical results characterizing Ctp1 variants with impaired DNA-binding or -bridging properties were consistent with Ctp1-mediated DNA bridging requiring the intact and correctly folded Ctp1 tetramer. Furthermore, mutations altering Ctp1 oligomerization and DNA bridging in vitro conferred cell sensitivity to DSB-producing agents. Together, these results support an important role for Ctp1-regulated DNA strand coordination required for DNA DSB repair in S. pombe.
2018
Coordinated protein and DNA conformational changes govern mismatch repair initiation by MutS
& Nucleic Acids Research
MutS homologs identify base-pairing errors made in DNA during replication and initiate their repair. In the presence of adenosine triphosphate, MutS induces DNA bending upon mismatch recognition and subsequently undergoes conformational transitions that promote its interaction with MutL to signal repair. In the absence of MutL, these transitions lead to formation of a MutS mobile clamp that can move along the DNA. Previous single-molecule FRET (smFRET) studies characterized the dynamics of MutS DNA-binding domains during these transitions. Here, we use protein–DNA and DNA–DNA smFRET to monitor DNA conformational changes, and we use kinetic analyses to correlate DNA and protein conformational changes to one another and to the steps on the pathway to mobile clamp formation. The results reveal multiple sequential structural changes in both MutS and DNA, and they suggest that DNA dynamics play a critical role in the formation of the MutS mobile clamp. Taking these findings together with data from our previous studies, we propose a unified model of coordinated MutS and DNA conformational changes wherein initiation of mismatch repair is governed by a balance of DNA bending/unbending energetics and MutS conformational changes coupled to its nucleotide binding properties.
Regulatory control of DNA end resection by Sae2 phosphorylation
Elda Cannavo, Dominic Johnson, Sara N. Andres, Vera M. Kissling, Julia K. Reinert, Valerie Garcia, Dorothy A. Erie, Daniel Hess, Nicolas H. Thomä, Radoslav I. Enchev, Matthias Peter, R. Scott Williams, Matt J. Neale, & Petr Cejka, Nature Communications
DNA end resection plays a critical function in DNA double-strand break repair pathway choice. Resected DNA ends are refractory to end-joining mechanisms and are instead channeled to homology-directed repair. Using biochemical, genetic, and imaging methods, we show that phosphorylation of Saccharomyces cerevisiae Sae2 controls its capacity to promote the Mre11-Rad50-Xrs2 (MRX) nuclease to initiate resection of blocked DNA ends by at least two distinct mechanisms. First, DNA damage and cell cycle-dependent phosphorylation leads to Sae2 tetramerization. Second, and independently, phosphorylation of the conserved C-terminal domain of Sae2 is a prerequisite for its physical interaction with Rad50, which is also crucial to promote the MRX endonuclease. The lack of this interaction explains the phenotype of rad50S mutants defective in the processing of Spo11-bound DNA ends during meiotic recombination. Our results define how phosphorylation controls the initiation of DNA end resection and therefore the choice between the key DNA double-strand break repair mechanisms.
We FRET so You Don’t Have To: New Models of the Lipoprotein Lipase Dimer
Cassandra K. Hayne, Hayretin Yumerefendi, Lin Cao, Jacob W. Gauer, Michael J. Lafferty, Brian Kuhlman, Dorothy A. Erie, & Saskia B. Neher, Biochemistry
Lipoprotein lipase (LPL) is a dimeric enzyme that is responsible for clearing triglyceride-rich lipoproteins from the blood. Although LPL plays a key role in cardiovascular health, an experimentally derived three-dimensional structure has not been determined. Such a structure would aid in understanding mutations in LPL that cause familial LPL deficiency in patients and help in the development of therapeutic strategies to target LPL. A major obstacle to structural studies of LPL is that LPL is an unstable protein that is difficult to produce in the quantities needed for nuclear magnetic resonance or crystallography. We present updated LPL structural models generated by combining disulfide mapping, computational modeling, and data derived from single-molecule Förster resonance energy transfer (smFRET). We pioneer the technique of smFRET for use with LPL by developing conditions for imaging active LPL and identifying positions in LPL for the attachment of fluorophores. Using this approach, we measure LPL–LPL intermolecular interactions to generate experimental constraints that inform new computational models of the LPL dimer structure. These models suggest that LPL may dimerize using an interface that is different from the dimerization interface suggested by crystal packing contacts seen in structures of pancreatic lipase.
2017
Using Atomic Force Microscopy to Characterize the Conformational Properties of Proteins and Protein–DNA Complexes That Carry Out DNA Repair
Sharonda J. LeBlanc, Hunter Wilkins, Zimeng M., Li, Parminder Kaur, Hong Wang, & Dorothy A. Erie, Methods in Enzymology
Atomic force microscopy (AFM) is a scanning probe technique that allows visualization of single biomolecules and complexes deposited on a surface with nanometer resolution. AFM is a powerful tool for characterizing protein–protein and protein–DNA interactions. It can be used to capture snapshots of protein–DNA solution dynamics, which in turn, enables the characterization of the conformational properties of transient protein–protein and protein–DNA interactions. With AFM, it is possible to determine the stoichiometries and binding affinities of protein–protein and protein–DNA associations, the specificity of proteins binding to specific sites on DNA, and the conformations of the complexes. We describe methods to prepare and deposit samples, including surface treatments for optimal depositions, and how to quantitatively analyze images. We also discuss a new electrostatic force imaging technique called DREEM, which allows the visualization of the path of DNA within proteins in protein–DNA complexes. Collectively, these methods facilitate the development of comprehensive models of DNA repair and provide a broader understanding of all protein–protein and protein–nucleic acid interactions. The structural details gleaned from analysis of AFM images coupled with biochemistry provide vital information toward establishing the structure–function relationships that govern DNA repair processes.
Substrate preference of Gen endonucleases highlights the importance of branched structures as DNA damage repair intermediates
Nucleic Acids Research
Human GEN1 and yeast Yen1 are endonucleases with the ability to cleave Holliday junctions (HJs), which are proposed intermediates in recombination. In vivo, GEN1 and Yen1 function secondarily to Mus81, which has weak activity on intact HJs. We show that the genetic relationship is reversed in Drosophila, with Gen mutants having more severe defects than mus81 mutants. In vitro, DmGen, like HsGEN1, efficiently cleaves HJs, 5΄ flaps, splayed arms, and replication fork structures. We find that the cleavage rates for 5΄ flaps are significantly higher than those for HJs for both DmGen and HsGEN1, even in vast excess of enzyme over substrate. Kinetic studies suggest that the difference in cleavage rates results from a slow, rate-limiting conformational change prior to HJ cleavage: formation of a productive dimer on the HJ. Despite the stark difference in vivo that Drosophila uses Gen over Mus81 and humans use MUS81 over GEN1, we find the in vitro activities of DmGen and HsGEN1 to be strikingly similar. These findings suggest that simpler branched structures may be more important substrates for Gen orthologs in vivo, and highlight the utility of using the Drosophila model system to further understand these enzymes.